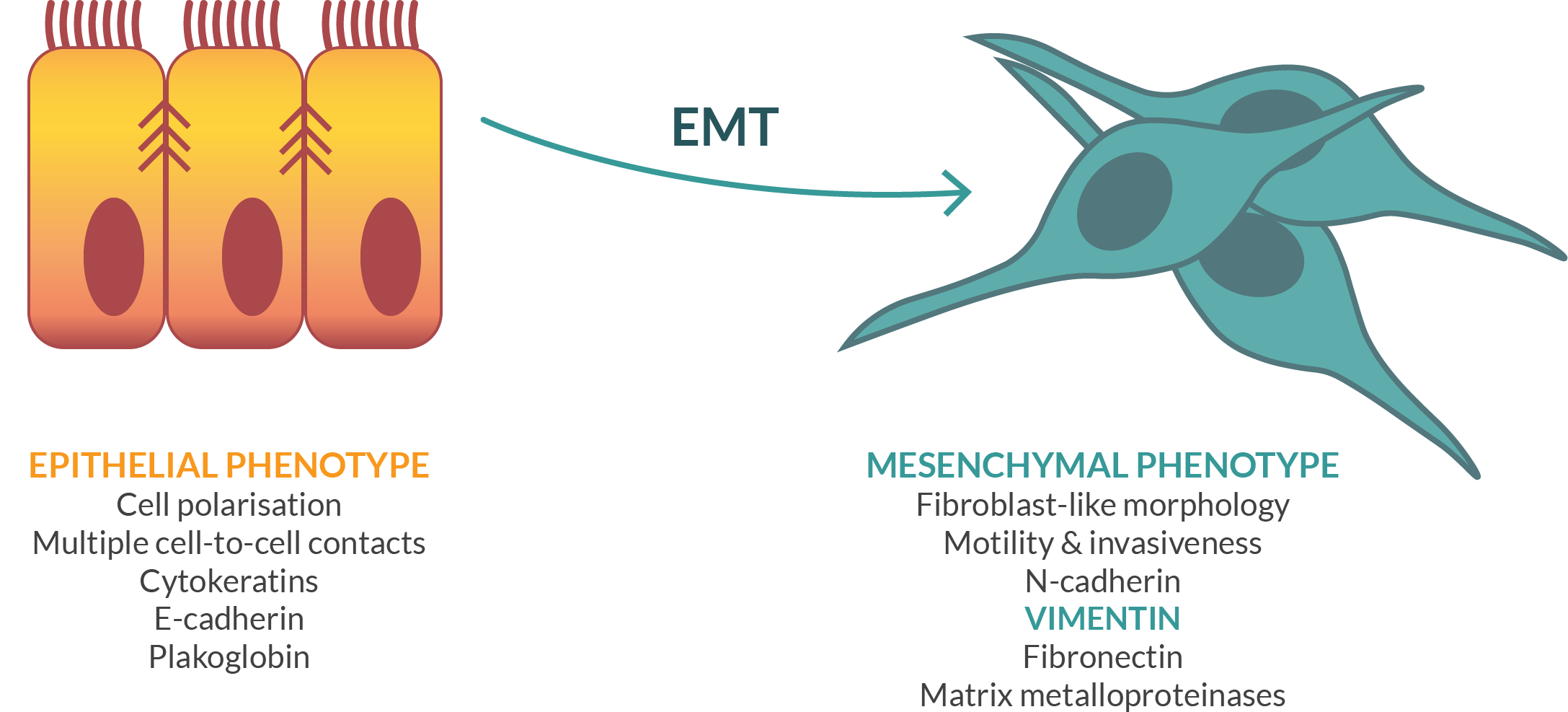
Modi-1 is the first vaccine based on Scancell’s Moditope® platform. The vaccine is composed of a combination of three peptides from two target antigens that are commonly modified in cancer cells. The first is the cytoskeletal protein, vimentin, which is preferentially digested during autophagy. All mesenchymal tumours such as endometrial, renal, sarcomas, lymphomas and lung tumours express vimentin as their major cytoskeletal protein and are potential targets for a modified vimentin vaccine. In addition, many epithelial tumours, such as breast, ovarian, gastrointestinal and prostate switch from expression of cytokeratin to vimentin during metastasis in a process known as epithelial mesenchymal transition (EMT). This change in phenotype enables the cell to become mobile and metastasize to new locations in the body.
The second target protein is the metalloenzyme alpha-enolase (α-enolase), which is involved in the process of glycolysis. Many tumours tend to favour metabolism via glycolysis even in normal oxygen conditions (the “Warburg effect”) and generate energy by converting pyruvate into lactate. The α-enolase enzyme mediates the penultimate step in this metabolic process and is overexpressed in a variety of cancers.
Modi-1 comprises three citrullinated peptides, two derived from vimentin and one from α-enolase; peptides from two different proteins have been combined in this way to reduce the possibility of tumour escape. In order to translate the Modi-1 peptides into the clinical setting, they have each been conjugated to a toll-like receptor (TLR) 1/2 agonist (AMPLIVANT®), which acts as an adjuvant. Scancell has entered into a worldwide licensing and collaboration agreement with ISA Pharmaceuticals (Leiden, the Netherlands) to use the AMPLIVANT® adjuvant technology for the development and commercialisation of Modi-1. Potent T cell responses and strong anti-tumour activity have been observed in several cancer models of different tumour types, including melanoma, ovarian, lung, pancreatic and triple negative breast cancer, following administration of the Modi-1 vaccine.
The first-in-human clinical study of Modi-1 is designed to explore the safety, immunological activity and preliminary efficacy of the vaccine in patients with solid tumours, including those with advanced head and neck, ovarian, triple-negative breast and renal carcinomas. Modi-1 will be administered in combination with checkpoint inhibitors in patients with head and neck, triple-negative breast or renal tumours [NCT05329532].