Immunobody®
ImmunoBody® DNA active immunotherapies encode a protein in the form of a modified antibody, which is engineered to express epitopes from a cancer antigen whilst retaining the ability to target activated antigen presenting cells in vivo.
iSCIB1+ is Scancell’s lead ImmunoBody®
iSCIB1+ is a needle-free tumour-targeted off-the-shelf immunotherapy selected for development in an upcoming registrational Phase 2-3 trial for advanced melanoma in combination with CPIs.
iSCIB1+ encodes a protein in the form of a modified antibody, which is engineered to express gp100 and TRP-2 epitopes whilst retaining the ability to target activated antigen presenting cells in vivo. By doing so, iSCIB1+ activates high-avidity T cells to generate a potent, lasting immune response, setting a new benchmark in the treatment of advanced metastatic melanoma. Around 80% of advanced melanoma patients have target HLA haplotypes.
iSCIB1+ and its earlier version SCIB1, with around half the melanoma-specific epitopes, have both demonstrated durable potent anti-tumour responses in combination with checkpoint inhibitors (CPIs) in SCOPE, an ongoing Phase 2 trial. SCIB1 has also demonstrated efficacy as a monotherapy in patients with adjuvant melanoma.
Supported by a robust GMP manufacturing process and a stable shelf life, iSCIB1+ offers a fast route to treatment for patients.
TUMOUR TARGETED
iSCIB1+ ImmunoBody® incorporates specific epitopes from the proteins gp100 and TRP-2 which play key roles in the production of melanin in the skin and were identified from T cells of patients who achieved spontaneous recovery from melanoma skin cancers.
DUAL ACTION
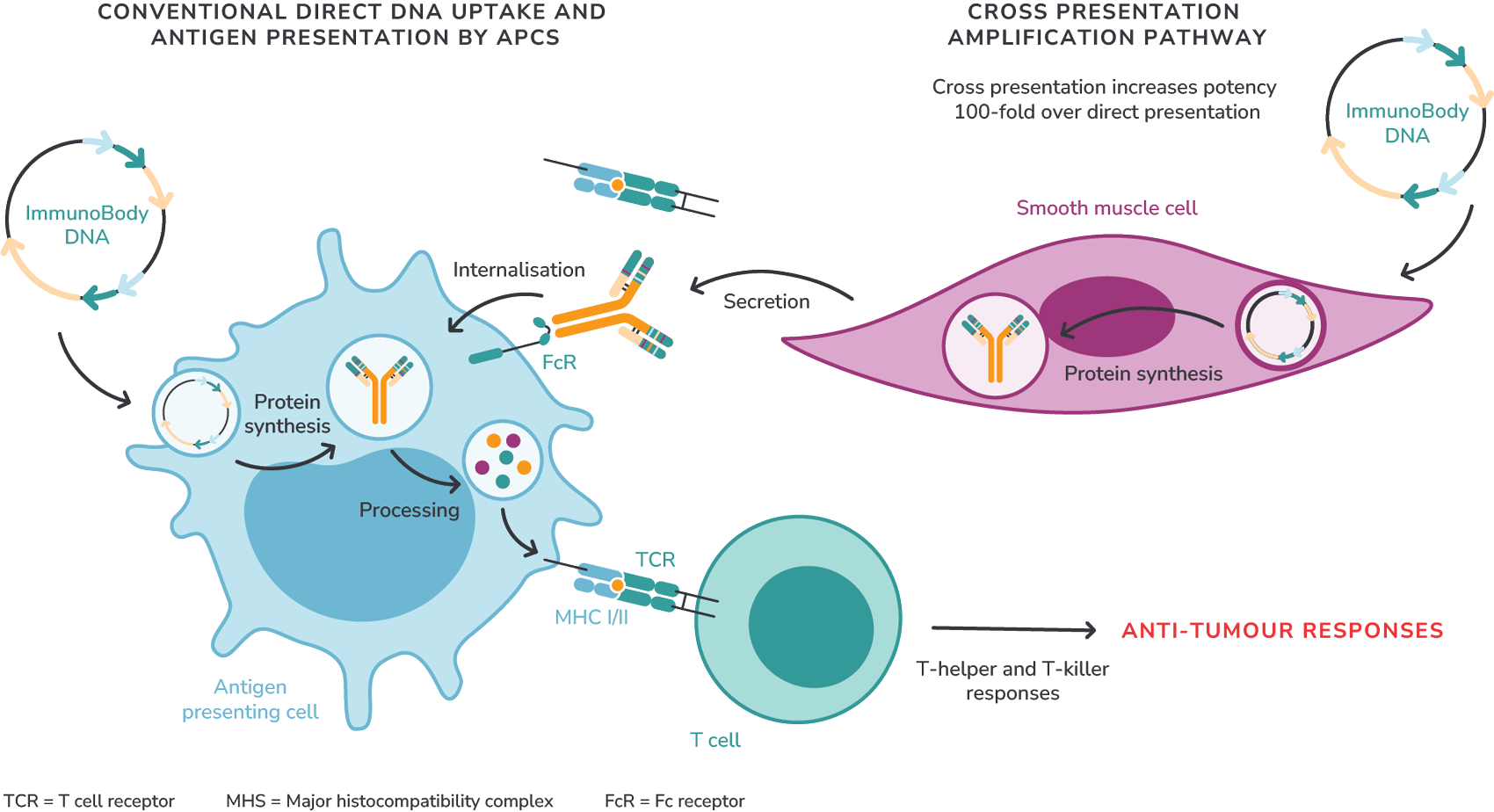
Direct and indirect Fc targeting of activated dendritic cells initiates direct and cross-presentation of epitopes to T cells resulting in higher T cell avidity of up to 100-fold increased potency and increased number of T cells to tumour epitopes.
LOW TOXICITY
Favorable safety and tolerability alone or when added to checkpoint inhibitor treatment, with potent vaccine specific T cell responses.

Targets antigen presenting cells in vivo to give potent T cell responses, attacking cancer on multiple fronts.
Robust GMP manufacturing process, stable shelf life and faster route to treatment allowing pricing flexibility. Five-year stability.
Delivers a spring-powered injection in 0.1 seconds by means of a narrow stream of fluid that penetrates the skin with a precise dose and depth.
Favourable safety profile when administered as a monotherapy and in combination with checkpoint inhibitors.
DNA targets can be adapted to target other cancers. Groundbreaking science leads to validated preclinical results and rapid entry into the clinic.
JOIN OUR MAILING LIST
Sign up for news updates to stay up to date with Scancell.