
During the development of the anti-glycan monoclonal antibodies (mAbs), Scancell identified unique sequence residues in the Fc region that enable mAbs to self-associate upon target recognition, resulting in more potent, high avidity antibodies.
MAbs are generated following the immunisation of mice with specific antigens. However, these are not in themselves ideal for use in humans as the host recognises the mouse antibody as foreign and will generate anti-mouse antibodies, limiting the effectiveness of the drug and potentially creating serious side effects. To overcome these hurdles, most therapeutic mAbs are produced as either chimeric antibodies (where the constant part of the antibody is replaced with the human sequence), as humanised antibodies (where only the antigen-binding regions of the murine antibody are retained) or as fully human antibodies.
During the development of Scancell’s anti-glycan mAbs, Scancell identified a number of key residues within the murine version of the mAbs that are responsible for inter-molecular cooperativity between the antibody molecules, which results in enhanced functional affinity and direct cell killing. Transfer of these murine residues to human Fc versions of the anti-glycan mAbs resulted in the promotion of non-covalent Fc-Fc interactions that enhanced the direct tumour cell killing ability of these mAbs. This forms the basis of the AvidiMab® technology platform.
Scancell’s high affinity anti-glycan mAbs are able to directly lyse tumour cells without the need for the complement system or immune effector cells. This is a form of inflammatory cell death and has the potential to initiate an anti-tumour immune response following the secretion of damage-associated molecular patterns (DAMPs), leading to long-term tumour control. Application of the AvidiMab® technology to Scancell’s anti-glycan mAbs increases non-covalent interactions between target-bound anti-glycan mAb molecules resulting in improved direct cell killing.
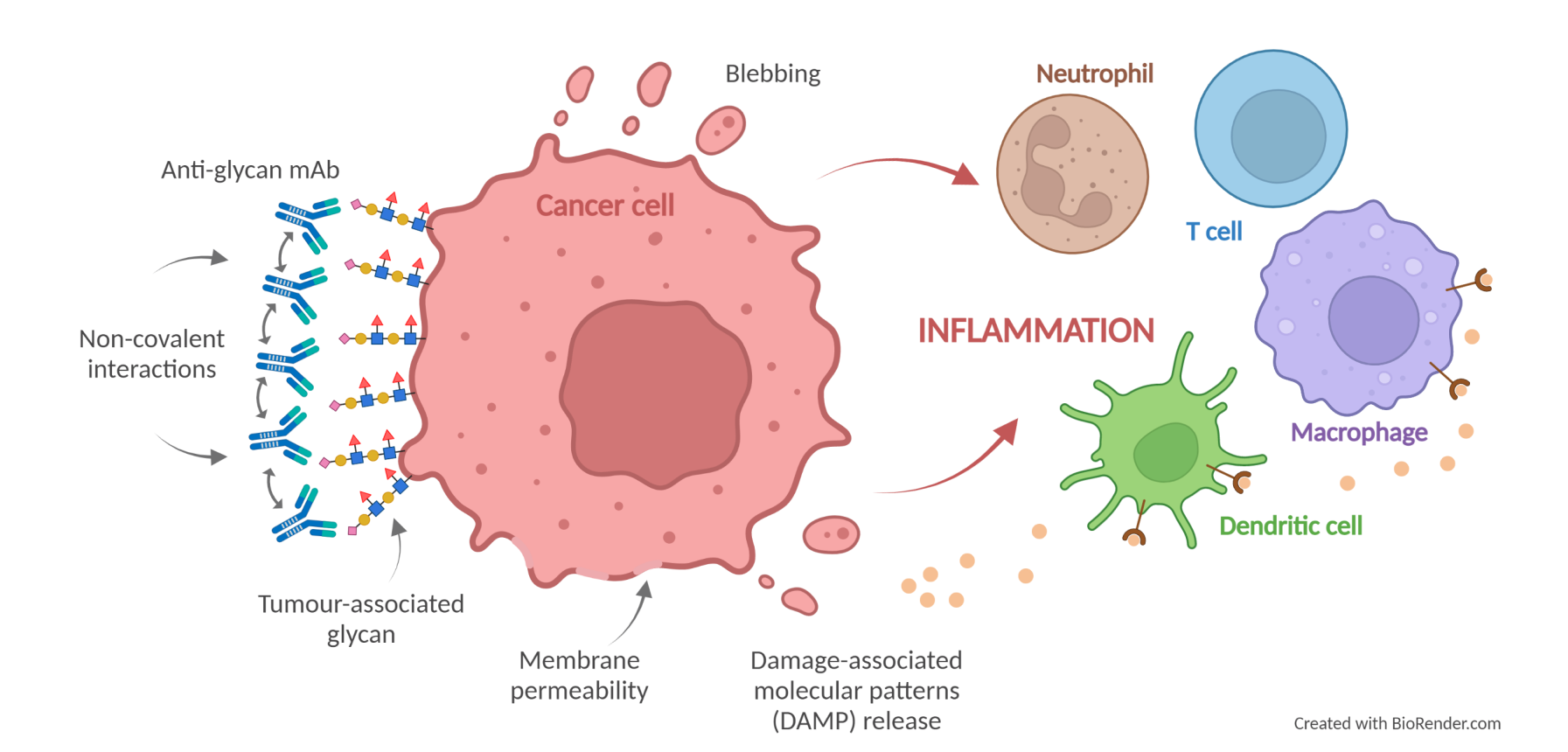
The AvidiMab® technology platform is patent-protected and, ultimately, could be applied to enhance the efficacy of potentially any chimeric or humanised mAb.
In addition, the AvidiMab® modifications have also been incorporated into the ImmunoBody® products iSCIB1+ and iSCIB2, and have also been included in the COVIDITY vaccine. In every instance, the modifications have enhanced the preclinical efficacy of these products.