
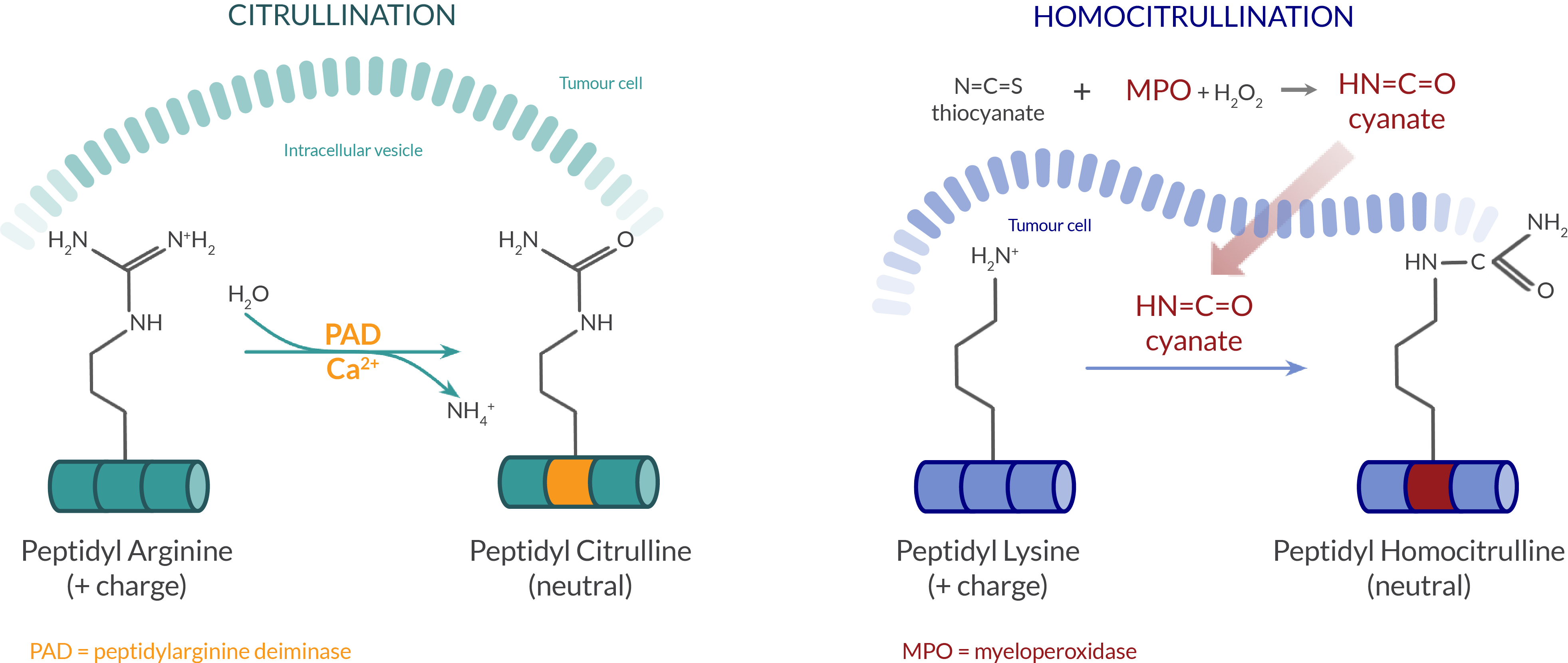
The Moditope® platform is based on exploiting the normal immune responses that remove stressed cells and represents a unique class of potent vaccines that is characterised by the induction of CD4 cytotoxic T cells. The nature of tumours means that most cancer cells live in stressful conditions that are often hypoxic and nutrient deficient. To survive in this environment, cancer cells often undergo autophagy, a process that allows cells to generate energy from the digestion of intracellular proteins and organelles. During this process stress-induced post-translational modifications (siPTMs) and proteolytic cleavage occurs resulting in the generation of peptides which can be presented on the cell surface complexed with major histocompatibility class II (MHC-II) molecules for recognition by T cells. However, cancer cells do not normally express MHC class II on their cell surface unless this is induced by inflammation. Examples of such modifications are citrullination, an enzyme-based conversion of arginine to citrulline, and homocitrullination (or carbamylation), in which lysine residues are converted to homocitrulline.
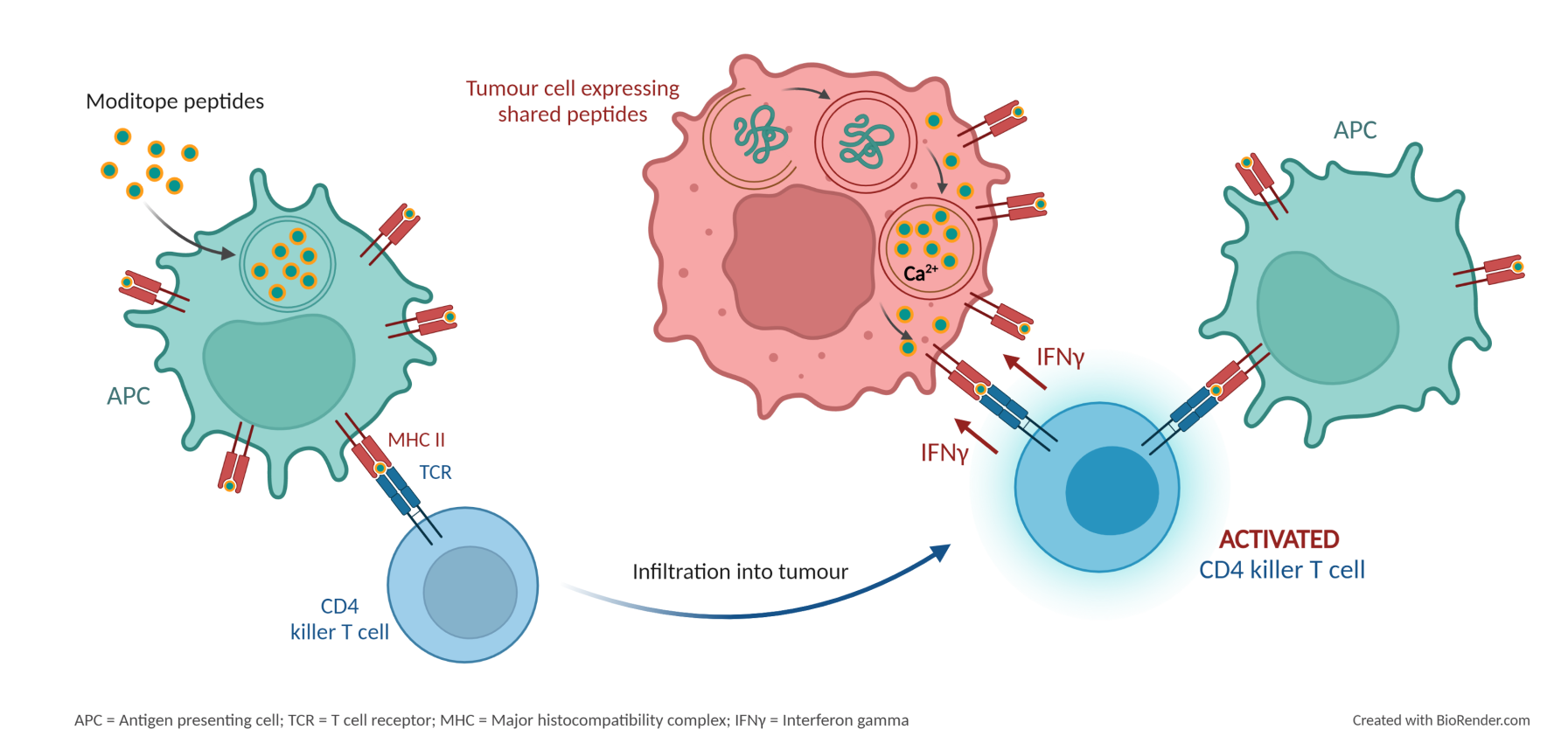
After vaccination with citrullinated or homocitrullinated peptides, the peptides are taken up by antigen presenting cells (APCs), such as dendritic cells, which process the peptides and present them to CD4 T cells. As these CD4 T cells infiltrate into the tumour microenvironment, they encounter modified peptides expressed on the surface of APCs and, as a result, the CD4 T cells become activated and secrete interferon-gamma (IFNγ) which induces inflammation and the up-regulation of MHC class II. The activated CD4 T cells can then see the modified peptides presented on MHC class II and directly kill the tumour cells.
Scancell currently has two Moditope® vaccines in clinical and preclinical development:
- Modi-1, a vaccine based on citrullinated peptides for the treatment of solid tumours including triple negative breast, ovarian, renal and head & neck cancers;
- Modi-2, utilises homocitrullinated peptides to address different cancer indications to Modi-1, including tumours with a particularly immunosuppressive environment.