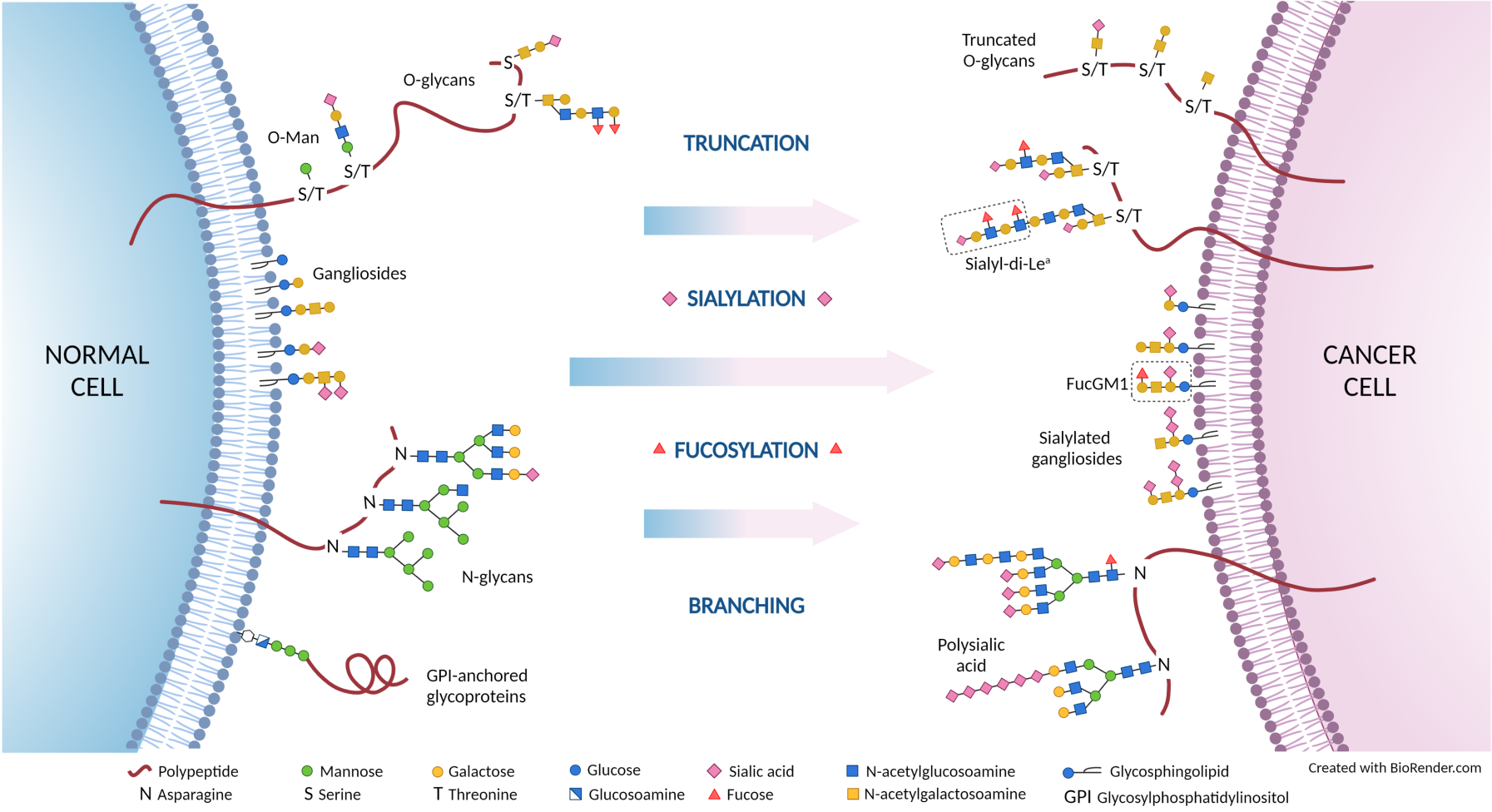
Glycosylation is a post-translational modification that occurs inside the cell and results in the addition of sugar motifs, “glycans”, to proteins and lipids that are, in most cases, destined for the cell surface. These glycan structures form the “glycome” and play an integral role in cell-to-cell and cell-to-matrix interactions through modulation of adhesion and cell trafficking. Glycosylation is increasingly recognised as a modulator of the malignant phenotype of cancer cells, where the interaction between cells and the tumour micro-environment is altered to facilitate processes such as drug resistance, metastasis and immune evasion. Antibodies recognising tumour-associated glycans could therefore have excellent therapeutic potential.
Scancell has used its GlyMab® technology to generate a series of high affinity mAbs targeting glycans that are over-expressed on cancer cells. The portfolio currently includes five mAb candidates; four targeting different cancers and one T-cell targeting antibody. Each mAb has high specificity for particular glycan molecules, making them attractive development candidates. Although Scancell’s initial focus is on pancreatic, lung, colorectal and gastric cancers, the individual glycans are also expressed on other tumour types which would broaden their potential utility to other indications.
- SC129: specific for the sialyl-di-Lewisa glycan, a target for pancreatic cancer
- SC134: specific for fucosyl GM1, a target for small cell lung cancer
- SC88: specific for the Lewisacx glycan, a target for colorectal cancer
- SC27: specific for the Lewisy glycan, a target for gastric cancer
- SC2811: specific for SSEA4 on human and mouse T cells with stem-like properties – a target for any solid tumour
Application of the AvidiMab® modifications to enhance non-covalent interactions between target-bound antibody molecules renders the antibodies capable of direct cell killing without the need for the complement system or immune effector cells, thereby increasing their potency.
In addition to being potential therapies in their own right, the specificity of the anti-glycan mAbs enables their development into a range of antibody-based therapies with differing mechanisms of action, including:
- Antibody drug conjugates (ADC) for the delivery of cytotoxic drugs, immunotoxins or radionuclide agents.
- Redirection of T cells using T cell - engaging bispecific antibodies to enhance immune-mediated anti-cancer therapeutic effects.
- A chimeric antigen receptor (CAR) approach for adoptive cell transfer.
Good targets for solid tumours have been an issue for CAR-based cell therapies or redirected T cell therapies in the past, resulting in unfavourable or even fatal toxicities. Scancell’s highly specific anti-glycan mAbs offer the potential to avoid these on-target/off-tumour specificity issues.
With regard to delivering cytotoxic payloads as ADCs, all of Scancell’s anti-glycan mAbs have shown effective internalisation, a key requisite for most ADCs in development. Increased internalisation of the anti-glycan mAb will result in a more effective payload delivery into the tumour cell and an enhanced anti-tumour effect.
The versatility and utility of Scancell’s current anti-glycan mAb portfolio, combined with the potential for continued expansion using the enabling GlyMab® technology platform and the potential application of the AvidiMab® technology to any mAb, creates wide-ranging co-development and licencing opportunities.