Scancell’s lead ImmunoBody® cancer vaccine, SCIB1, is being developed for the treatment of patients with metastatic melanoma. SCIB1 incorporates specific epitopes from the proteins gp100 and TRP-2, which were identified from the cloning of T cells from patients who achieved spontaneous recovery from melanoma skin cancers. Both proteins play key roles in the production of melanin in the skin.
SCIB1-001
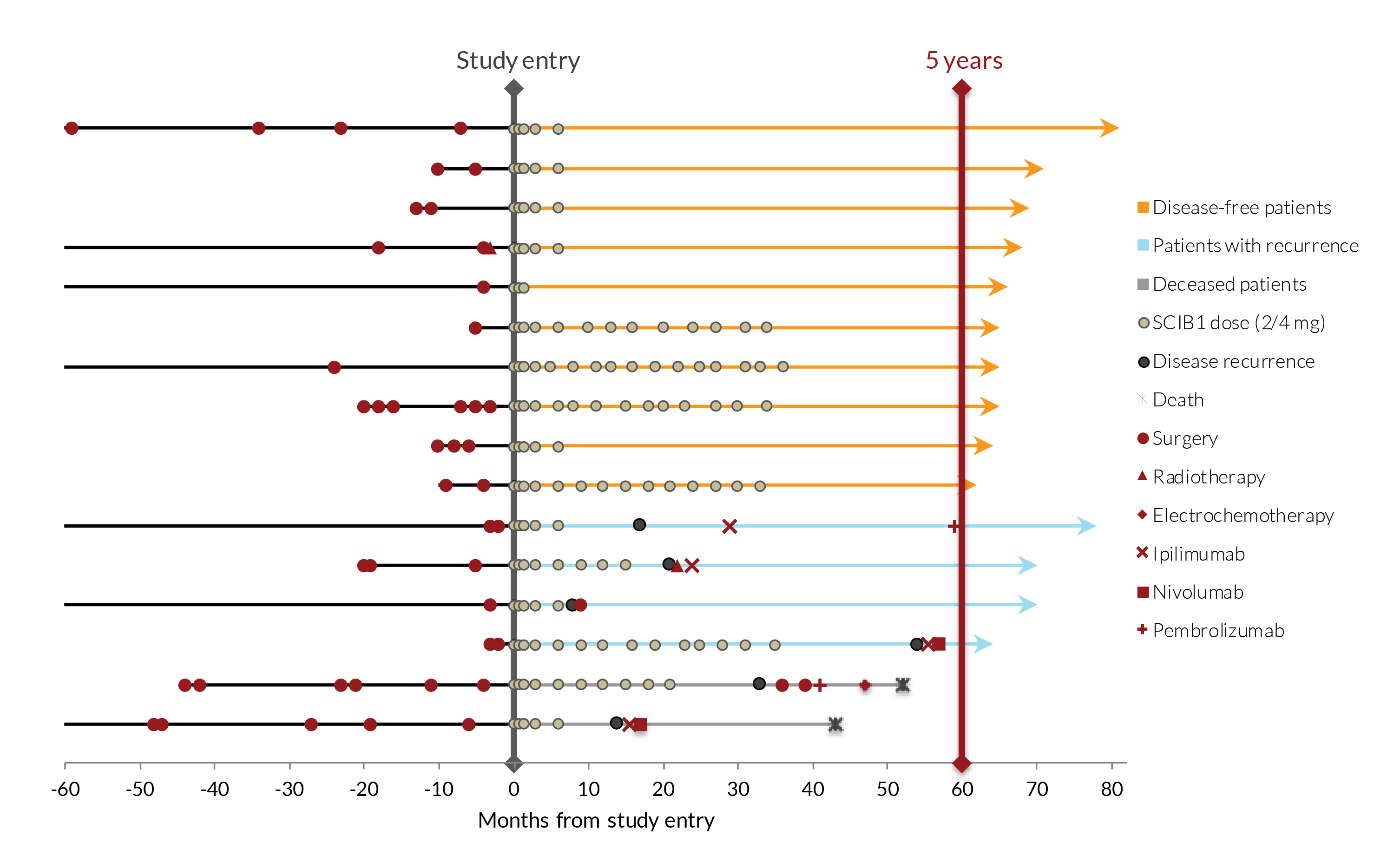
A Phase 1/2 study of SCIB1 in 35 patients with stage III or IV disease, in which 15 patients had tumours present and 20 had fully-resected disease at study entry has been successfully completed. Dose-dependent T cell responses were induced in nearly 90% of patients with no serious adverse events or dose limiting toxicities. At the data cut-off point for the main study, all 20 fully-resected patients were alive, with a median observation time of 37 months from study entry. In the 16 patients with fully-resected disease who received 2-4 mg doses of SCIB1, 14 were still alive 5 years after the study had started. The melanoma recurrence rates in resected SCIB1-treated patients were also lower than in historical controls.
SCIB1-002
Immune checkpoint inhibitors (CPIs) such as Keytruda® (pembrolizumab) and Opdivo® (nivolumab) relieve the immunosuppression present in the tumour microenvironment, which can affect an individual’s immune response to their cancer. There is a clear rationale for using an ImmunoBody® to prime an immune response against a tumour to enhance the efficacy of such CPIs. This potential has been confirmed in preclinical studies, which indicate that SCIB1 and an anti-PD1 antibody CPI have similar activity levels as monotherapies and have a strong synergistic effect when administered together. The combination of CPI treatment with a therapeutic vaccine has the potential to increase the proportion of patients who respond to the combined treatment. This hypothesis is being tested in Scancell’s Phase 2 clinical study of SCIB1 in late-stage melanoma patients receiving Keytruda® as standard of care [NCT04079166].
Further information about the trial can be found here.
iSCIB1+
The SCIB1 ImmunoBody® expresses a select number of melanoma-specific T cell epitopes that restricts its potential efficacy to approximately 40% of patients. The modified vector SCIB1+ includes the same epitopes as SCIB1 but now also includes several more epitopes to overcome this limitation. In a further modification, the Fc region of the SCIB1+ product was improved using the AvidiMab® technology, generating the iSCIB1+ DNA vector, to enhance the Fc targeting of the ImmunoBody® to dendritic cells resulting in the induction of higher frequency T cell responses.
SCOPE Clinical Advisory Board